New polymer electrolyte for lithium-metal batteries – pv magazine International
Researchers on the College of Hong Kong have found a collection of anionic community stable electrolytes that may present a minimum of a fourfold enchancment in cationic transport for lithium-metal battery purposes.
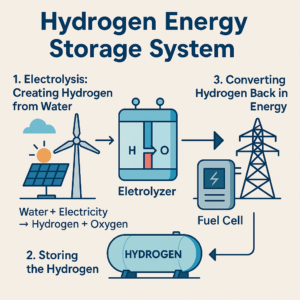
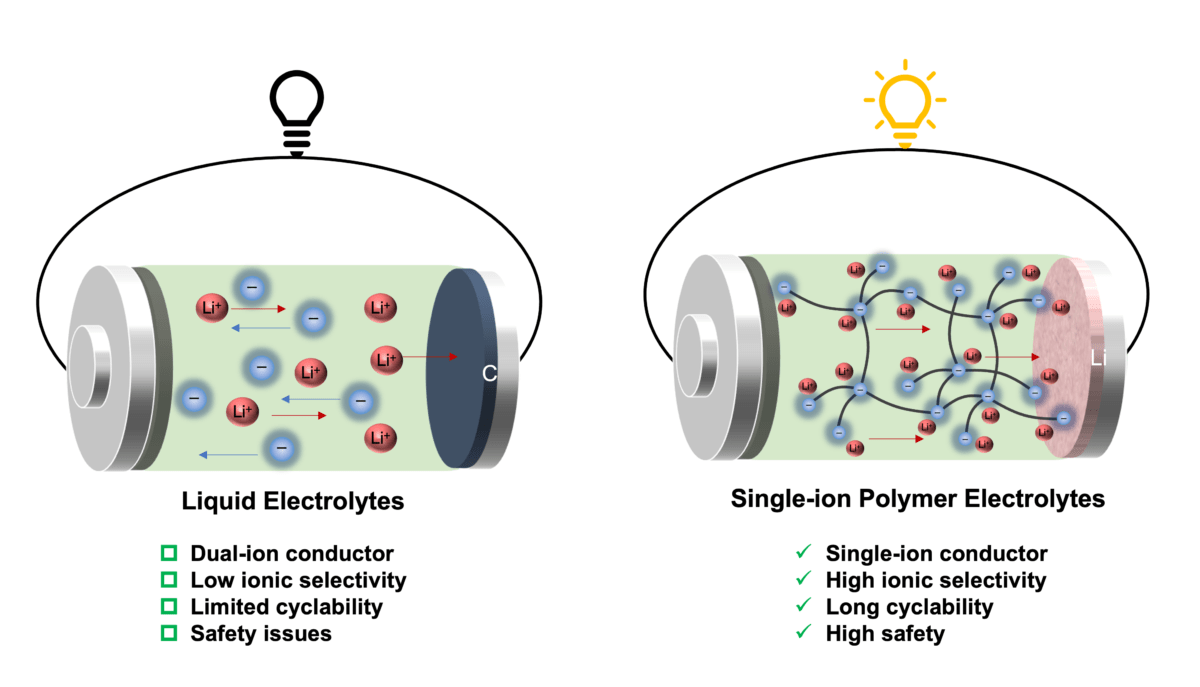
Whereas lithium metallic batteries supply higher security, higher vitality density, and decrease weight than lithium-ion know-how – due to changing heavier graphite with lithium metallic because the anode – this battery chemistry doesn’t work properly with standard electrolytes.
Due to this fact, analysis efforts are directed in direction of stable electrolytes that present higher efficiency and are appropriate with the lithium metallic anode, which at the moment exhibits the very best theoretical particular energy capability.
Now, researchers on the College of Hong Kong (HKU) have engineered solvent-free single-ion polymer electrolytes that present important enhancements in ionic transport for lithium-metal battery purposes.
That’s, in liquid electrolytes, lithium cations and counter anions transfer in reverse instructions to conduct electrical energy. Usually, anions transfer a minimum of 4 occasions quicker than lithium cations, and due to this fact the switch of lithium cations contributes solely a small half (20%) of the overall ionic present. As extra anions accumulate on the interface between the electrode and electrolyte, it causes inner quick circuits and capability loss within the battery.
New single-ion conducting polymer electrolytes designed by HKU researchers are reported to beat these limitations and result in a minimum of a fourfold enhance in cationic transport.
Anionic community polymers encompass borate anions linked by branched ethylene glycol linkers in numerous stoichiometric ratios, the place the anions are hooked up to the polymer body, enabling a selective cation transport.
The researchers managed the cation conductivity throughout the polymer via the systematic engineering of segmental mobility. This helped them to map complete design guidelines for a brand new class of extremely conductive stable electrolytes, which efficiently overcome the persistent issues of present stable electrolytes, akin to low cyclability and excessive which is overpotential.
“We imagine that single-ion conducting polymer electrolytes will open the potential of new battery chemistries that may revolutionize the sphere of rechargeable batteries, and supply excessive ranges of security, excessive energy density, and lengthy cycles in life,” mentioned Jingyi Gao, the primary writer of the analysis paper.
In accordance with Dr Dong-Myeong Shin of HKU, ion-selective electrolytes may lead to quick charging as a result of low overpotential. “This may allow electrical automobiles to be totally charged within the time it takes to drink a cup of espresso. This distinctive benefit will open a brand new period in a clear vitality world,” he added.
Their findings are mentioned in “Engineered networking of a household of solvent-free single-ion conducting borate community polymer electrolytes for Li-metal battery purposes” revealed in Journal of Chemical Engineering.
This content material is protected by copyright and is probably not reused. If you wish to cooperate with us and need to reuse a few of our content material, please contact: [email protected].