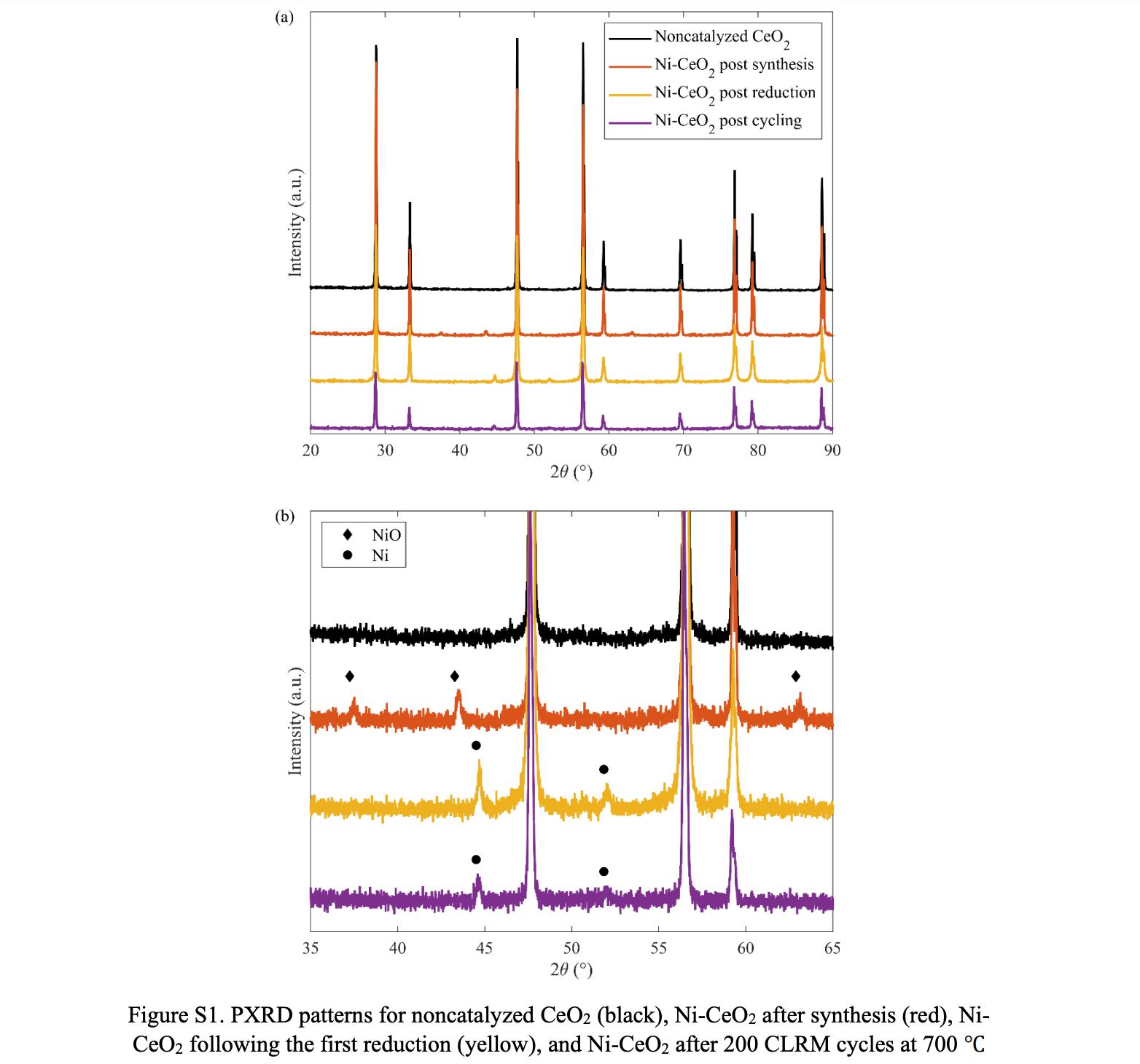
Published at Sustainable Energy & Fuels – Kinetic investigation of solar chemical looping reforming of methane over Ni–CeO2 at low temperature
Summary:
Utilizing photo voltaic thermal power to drive the chemical looping reforming of methane (CLRM) is a promising technique to effectively and selectively reform methane to provide syngas utilizing renewable power. On this work, the function of catalytically lively nickel in response kinetics, conversion, selectivity, and whole syngas manufacturing throughout CLRM of Ni-CeO2 investigated. By means of thermogravimetric evaluation (TGA), metallic nickel was proven to assist enhance the partial oxidation of methane (POM) response charges on account of the decrease activation power response mechanism in all oxygen nonstoichiometries, in comparison with CeO2. For instance, the discount of Ni-CeO2 at 700 °C is corresponding to CeO2 at 900 °C, and no response was noticed for CeO2 at 700 °C. Moreover, prolonged biking with Ni-CeO2 confirmed steady response charges and yields throughout CLRM at 700 °C, and SCo remained above 0.98 at some point of the experiment. The usage of a bigger packed-bed reactor system, Ni-CeO2 additionally confirmed comparable methane conversion, syngas manufacturing and selectivity to CeO2however at a lot decrease working temperatures, ie, Q ≤ 800 °C. A better price of coking was noticed throughout the POM of Ni-CeO2; nonetheless, all carbon was eliminated within the subsequent step and accumulation was not noticed throughout long-term biking. A parametric examine of gasoline velocity, temperature, and inlet partial strain of methane can also be offered to evaluate the impact of those working situations on conversion, selectivity, and syngas manufacturing. Notably, a tradeoff between the conversion and the quantity of syngas produced was noticed because the gasoline velocity elevated; nonetheless, the conversion time response signifies that there’s an excellent response cut-off time the place excessive syngas manufacturing charges may be concurrently achieved with near-complete methane conversion.